Proven Tolerability and Safety through week 21
Results were seen in multiple clinical trials
In randomized, multicenter, vehicle-controlled clinical trials, 351 adults with plaque psoriasis were treated with LEXETTE Foam twice daily for up to 2 weeks (up to a maximum of 50 grams per week).
Incidence of treatment-related adverse reactions in ≥1% of patients treated with LEXETTE Foam through Week 21
| Adverse reaction | LEXETTE FOAM N=351 |
Vehicle Foam N=353 |
|---|---|---|
| Application site burning/stinging | 12% | 15% |
| Application site pain | 1% | <1% |
| Headache | 1% | <1% |
Skin atrophy (n=1) and telangiectasia (n=2) were reported with LEXETTE Foam, but not with vehicle foam.
Adverse reactions were mostly mild in severity1
Patients prefer foam!
In 2 studies of 659 plaque psoriasis patients, 86% said they would comply with a foam treatment 75%–100% of the time.2
Patients treated with LEXETTE Foam stayed on treatment as directed*1

treated with LEXETTE Foam discontinued treatment due to adverse reactions
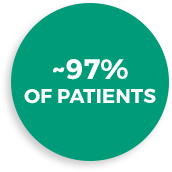
who used LEXETTE Foam complied with treatment for the entire treatment period
*A patient was considered compliant with the dosing regimen if the patient applied at least 80% of expected applications and did not have any significant dosing protocol deviations.

